By Joseph Haddad
Over the past year the Open Insulin chapter at Counter Culture Labs has focused on refining our techniques for using transgenic Pichia pastoris in order to synthesize glargine, a type of long acting insulin. In 2024 and 2025 there have been two flask culture runs and one bioreactor run that have been confirmed to have successful glargine production with protein gels and western blots.
The bioreactor run from 6/24/2024/ to 6/30/2024 has the most promising result because the other successful results were cultures grown in flasks which are less useful for producing large amounts of Insulin than cultures in a bioreactor. The synthesis pathway for glargine that was integrated into Pichia pastoris is methanol activated, meaning it will only produce glargine when methanol is added to the feedstock.
Figure 1: Protein gel from 10/12/2024, samples from the bioreactor run of 6/24/2024/ to 6/30/2024.
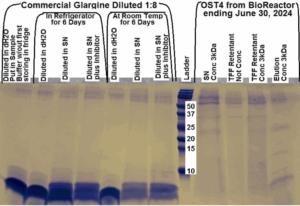
In Figure 1, the last lane from the right, saw a positive result for glargine expression. Glargine is a protein that consists of two chains; these chains are cleaved in commercial samples but remain uncleaved after it has been expressed and secreted by a cell. The column Elution Conc 3kDa, last lane from the right, showed a positive result for glargine expression at the expected 6kDa mark. This sample went through a concentration with a 3 kilodalton filter. The bioreactor samples that were not concentrated showed a negative result for glargine expression.
Figure 2: Western blot from 11/11/2024, samples from the bioreactor run of 6/24/2024/ to 6/30/2024.
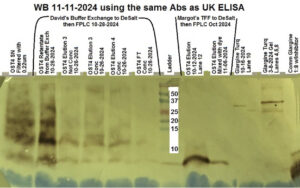
In Figure 2 the western blot uses antibodies that bind to glargine to detect it. In samples positive for glargine, the antibodies binding will stain the sample brown. The two samples to the right of the ladder, labeled “Margot’s TFF to DeSalt then FPLC Oct 2024” have bands at 6kDa that are stained brown, indicating glargine expression. These samples are from the bioreactor run of 6/24/2024/ to 6/30/2024, and were processed through a concentration with a 3 kilodalton filter. This test confirms the presence of the glargine detected in the protein gel in Figure 1.
The Open Insulin team at Counter Culture Labs has confirmed glargine expression from this bioreactor run as well as two flask runs. The first of these flask runs was grown from 11/25/2024 – 12/04/2024 and the second one was grown from 02/05/2025 – 02/24/2025. The former run was confirmed only via a protein gel; the latter run was confirmed via both a protein gel and a western blot.
Figure 3: Protein gel from 12/13/2024, samples from the flask run of 11/25/2024 – 12/04/2024.
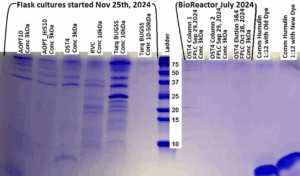
In Figure 3, the flask culture that was active from 11/25/2024 – 12/04/2024 showed positive results for glargine expression at 6 kDa in the third lane from the left. This sample was run through a 3 kilodalton filter. There were also samples from a bioreactor run in July 2024 that this gel tested which were unsuccessful.
Figure 4: Protein Gel from 2/24/2025, samples from the flask run of 02/05/2025 – 02/24/2025
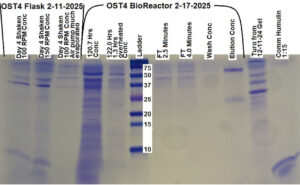
In Figure 4, the protein gel that was run on 2/24/2025 that showed positive results for glargine expression in two out of three samples taken from the flask culture that was active from 02/05/2025 – 02/24/2025.
Figure 5: Western blot from 05/23/2025, samples from the flask run of 02/05/2025 – 02/24/2025
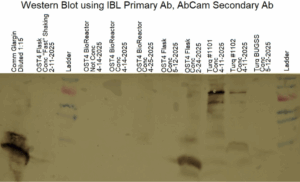
In order to further confirm glargine expression in the flask culture of 02/05/2025 – 02/24/2025 a western blot used antibodies that bind to glargine. In Figure 5, on the eighth lane from the left, a sample from this flasks culture shows antibodies identifying the presence of glargine. This confirms the synthesis of glargine by transgenic Pichia pastoris detected by Figure 4.
While these three cultures showed that the Pichia pastoris strain developed by Open Insulin is capable of expressing significant quantities of insulin glargine, expression is not consistent . The next step in our work is to continue experiments to obtain reliable and consistent expression.